background and overview[1]
3-chlorotoluene, also called m-chlorotoluene, is a colorless liquid mainly used in the manufacture of pesticides, medicines, dyes and peroxide intermediates and solvents. 3-chlorotoluene is a by-product of the chlorination of toluene to produce o-chlorotoluene and p-chlorotoluene, but its content is very low and separation is difficult. generally, 3-chlorotoluene is not prepared through chlorination of toluene.
synthesis method[1]
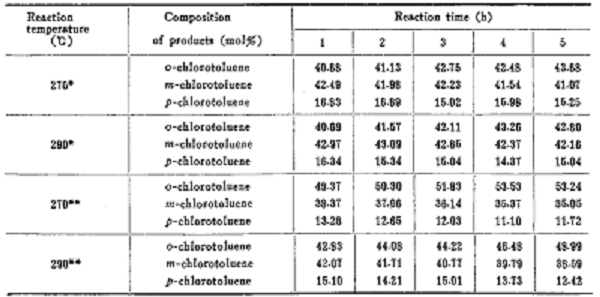
o-chlorotoluene is used as raw material and isomerized to prepare 3-chlorotoluene. use industrial grade o-chlorotoluene as the reaction raw material, analytically pure toluene and chlorobenzene as the diluent, and mix them evenly with o-chlorotoluene in a certain volume ratio. cylinders n2 and h2 are used as carrier gases. the isomerization reaction is carried out in a quartz tube integrating reactor, and the catalyst dosage is 7 to 8 grams. after the fresh catalyst was activated in the air at 500°c for 1 hour, it was lowered to the reaction temperature with n2, and fed into the reaction after constant temperature. the raw materials are transported by ultra-micro pumps, and the rapidly vaporized sample vapor is continuously brought into the catalyst bed by the carrier gas. the reaction products are condensed and collected according to a predetermined time. after the catalyst (hzsm-5) is coked and deactivated, it is regenerated by air at 500°c for 2 hours before being used for the reaction. the collected liquid product was analyzed by capillary chromatography. the product composition is shown in the figure below.
application fields[2][3]
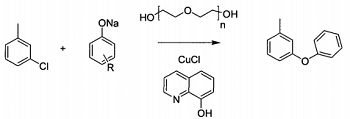
1. preparation of m-phenoxytoluene
stir and mix 3-chlorotoluene, sodium phenolate, cuprous chloride, 8-hydroxyquinoline and polydiethylene glycol at room temperature in a reaction vessel, and heat until the temperature of the mixture is 130~140°c, then keep it warm and stir the reaction lasts for 8 to 9 hours, and finally, through high vacuum and reduced pressure distillation, the 100 to 110°c fraction is taken to obtain m-phenoxytoluene. the reaction equation is as follows. in this reaction, sodium phenolate and 3-chlorotoluene are used as raw materials, and m-phenoxytoluene is reacted in the next step under the action of a catalyst. m-chlorotoluene is both a reactant and a solvent.
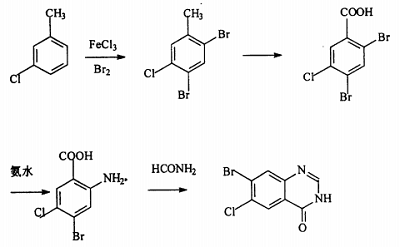
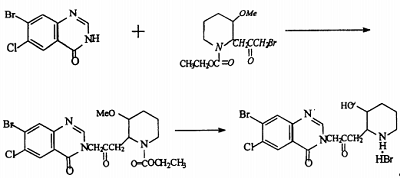
2. preparation of fentanyl hydrobromide
hydroxanone hydrobromide is 7-bromo-6-chloro-3-[3-(3-hydroxy-2-piperidinyl)-2-oxopropyl]-4(3h)-quinolinone , a natural alkaloid, was first used as a veterinary drug and has unique effects in fighting poultry coccidiosis. using 3-chlorotoluene as raw material and ferric chloride as catalyst, it undergoes bromination reaction with butane bromide and bromine to obtain 2,4-dibromo-5-chlorotoluene, which is produced by potassium permanganate, potassium dichromate or dichromate. magnesium oxide converts the methyl group into a carboxyl group to obtain 2,4-dibromo-5-chlorotoluic acid. it reacts with ammonia water or ammonia gas in the presence of cuprous chloride to selectively ammonate its ortho-bromine to obtain 2- methyl-4-bromo-5-chlorobenzoic acid is then cyclized in dmf solution using formamide to obtain 7-bromo-6-chloro-4(3h)-quinolinone, which is finally combined with 2-bromoacetonyl-3- methoxy-1-piperidinecarboxylic acid ethyl ester is condensed, hydrolyzed, and demethylated to prepare oftenanone hydrobromide. the reaction process is as follows.
main reference materials
[1] zhao zhenhua, lin lixi. study on the atmospheric pressure isomerization reaction of o-chlorotoluene over hzsm-5 zeolite catalyst[j]. acta catalytica sinica, 1987, 8: 430-435.
[2] jing weibi, lin ming, zhu gui, synthesis method of m-phenoxytoluene, cn 201010234546, application date 2010-07-19
[3] mao zhongxing, mao guanxing, jin junting, preparation method of fentanone hydrobromide, cn 200410016161, application date 2004-02-04


