background and overview[1]
1,4-dibromo-2-fluorobenzene can be used as an intermediate for pharmaceutical and chemical synthesis. if 1,4-dibromo-2-fluorobenzene is inhaled, move the patient to fresh air; if there is skin contact, take off contaminated clothing, wash the skin thoroughly with soap and water, and seek medical attention if you feel uncomfortable; if eye contact occurs, separate eyelids, rinse with running water or saline, and seek medical attention immediately; if ingested, rinse mouth immediately, do not induce vomiting, and seek medical attention immediately.
apply[1]
1,4-dibromo-2-fluorobenzene can be used as raw material to prepare a fluorine-substituted organic small molecule hole transport material. the synthesis route is as follows:
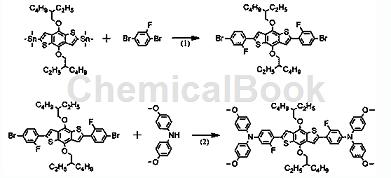
step 1: 1,1′-[4,8-bis[(2-ethylhexyl)oxy]benzo[1,2-b:4,5-b’]dithiophene under nitrogen conditions -2,6-diyl]bis[1,1,1-trimethyltin] (0.5g, 0.65mmol), 1,4-dibromo-2-fluorobenzene (1.0g, 3.9mmol), tetra( triphenylphosphine)palladium (pd(pph 3 ) 4 ) (22.5 mg, 19.5 μmol) and toluene (100 ml) were added to a 250 ml round-bottomed flask. react for 6 hours at 80°c. after the reaction is completed, cool to room temperature, extract with dichloromethane (ch 2 cl 2 ), wash the organic phase with brine, and anhydrous mgso 4 dry, evaporate and concentrate to remove the solvent. the crude product was chromatographed with ch 2 cl 2 and n-hexane column chromatography (hexane: ch 2 cl 2 =4 :1 (v/v)), obtaining a yellow-green intermediate product (270 mg, yield 53%).
step 2: nitrogen protection, the above intermediate (0.79g, 1mmol), 4,4′-dimethoxydiphenylamine (0.58g, 2.5mmol), tris(dibenzylideneacetone)dipalladium (pd 2 (dba) 3 ) (37mg, 0.12mmol), tri-tert-butylphosphine (p(t-bu) 3 ) (26mg , 0.12mmol), sodium tert-butoxide (naot-bu) (25mg, 0.25mmol) and toluene (20ml) were added to a 100ml round-bottom flask and reacted at 110°c for 2d (48 hours). after the reaction was completed, the mixture was cooled to room temperature. the mixture was extracted with dichloromethane (ch 2 cl 2 ) and washed with brine. the organic layer was dried with mgso4 and concentrated by rotary evaporation to remove the solvent. the crude product was chromatographed with ch 2 cl 2 and n-hexane column chromatography (hexane: ch 2 cl 2 =2 :1(v/v)). finally, a yellow powder (886 mg, yield 82%) was obtained.
main reference materials
[1] cn108467402 fluorine-substituted organic small molecule hole transport materials and their applications


