background and overview[1]
4-[3-(4-hydroxybutyl)-4,4-dimethyl-2,5-dioxo-1-imidazolidinyl]-2-(trifluoromethyl)benzonitrile used as pharmaceutical and chemical synthesis intermediates. if 4-[3-(4-hydroxybutyl)-4,4-dimethyl-2,5-dioxo-1-imidazolidinyl]-2-(trifluoromethyl)benzonitrile is inhaled, get move the patient to fresh air; in case of skin contact, take off contaminated clothing, rinse the skin thoroughly with soap and water, and seek medical attention if you feel uncomfortable; if the eye contact occurs, separate the eyelids and rinse with running water or normal saline rinse and seek medical attention immediately; if ingested, rinse mouth immediately. do not induce vomiting and seek medical attention immediately.
structure
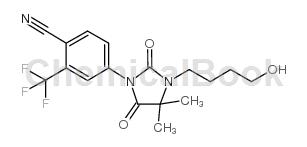
preparation [1]
method 1: 4-[3-(4-hydroxybutyl)-4,4-dimethyl-2,5-dioxo-1-imidazolidinyl]-2-(trifluoromethyl) the synthesis of benzonitrile is as follows:
step 1: synthesis of 4-amino-2-(trifluoromethyl)benzonitrile. under a nitrogen atmosphere, add 377 mg copper (ii) cyanide 14c (9gbq) and 1.0732g 4-bromo-3-(trifluoromethyl) into 8 ml dimethylformamide, mix, reflux for 4 hours, and cool to 0 ℃ and dilute with 20ml acetone. the insoluble portion was filtered off, and the filtrate was concentrated under reduced pressure at 700°c. the residue was dissolved in dichloromethane, filtered, and the filtrate was concentrated under reduced pressure. purification of benzonitrile (14c) by silica chromatography (eluent: dichloromethane-cyclohexane (70-30)) afforded 0.558 g (6.62 gbq) of the expected product.
step 2: synthesis of 4-isocyanato-2-(trifluoromethyl)benzonitrile. 182.4 mg of benzonitrile (14c) (0.97 mmol), 2 ml of dioxane and 1 ml of toluene of 20% phosgene were mixed together under a nitrogen atmosphere, and the solution was heated at 60 °c for 22 h and then at 60 decompress concentratedly at ℃. the isocyanate was used as such in the next step.
step 3: 4-(4,4-dimethyl-3-(4-hydroxybutyl)-5-imino-2-oxo-1-imidazolidinyl)-2-(trifluoromethyl base) benzonitrile synthesis. add 1.5ml dichloromethane (on siliceous rock nk 30), 4-(4,4-dimethyl-3-(4-hydroxybutyl)-5-imino-2-oxo- a solution of 1-imidazolidinyl)-2-(trifluoromethyl)-(5-3h)-propyl benzonitrile in 1.5 ml dichloromethane and 150 μl triethylamine, the isocyanate of step 2), and the mixture was stir at 20°c for 1 hour and concentrate under reduced pressure. the imine was used as such in the next step.
step 4. 4-[3-(4-hydroxybutyl)-4,4-dimethyl-2,5-dioxo-1-imidazolidinyl]-2-(trifluoromethyl) synthesis of benzonitrile. add 5 ml methanol and 1.2 ml 1n hydrochloric acid to the imine from step 3, reflux the mixture for 40 minutes, then reach 200°c again and dilute with 10 ml water. extract with dichloromethane, wash the extract with water and concentrate under reduced pressure. the crude product was purified by silica chromatography (eluent: diethyl ether-acetonitrile-cyclohexane (50-15-35)) to give 289 mg (1.26 gbq) of the expected product.
main reference materials
[1] wo2009097996.use of substituted phenylimidazolidines for producing medicaments for treating metabolic syndrome


